Introduction
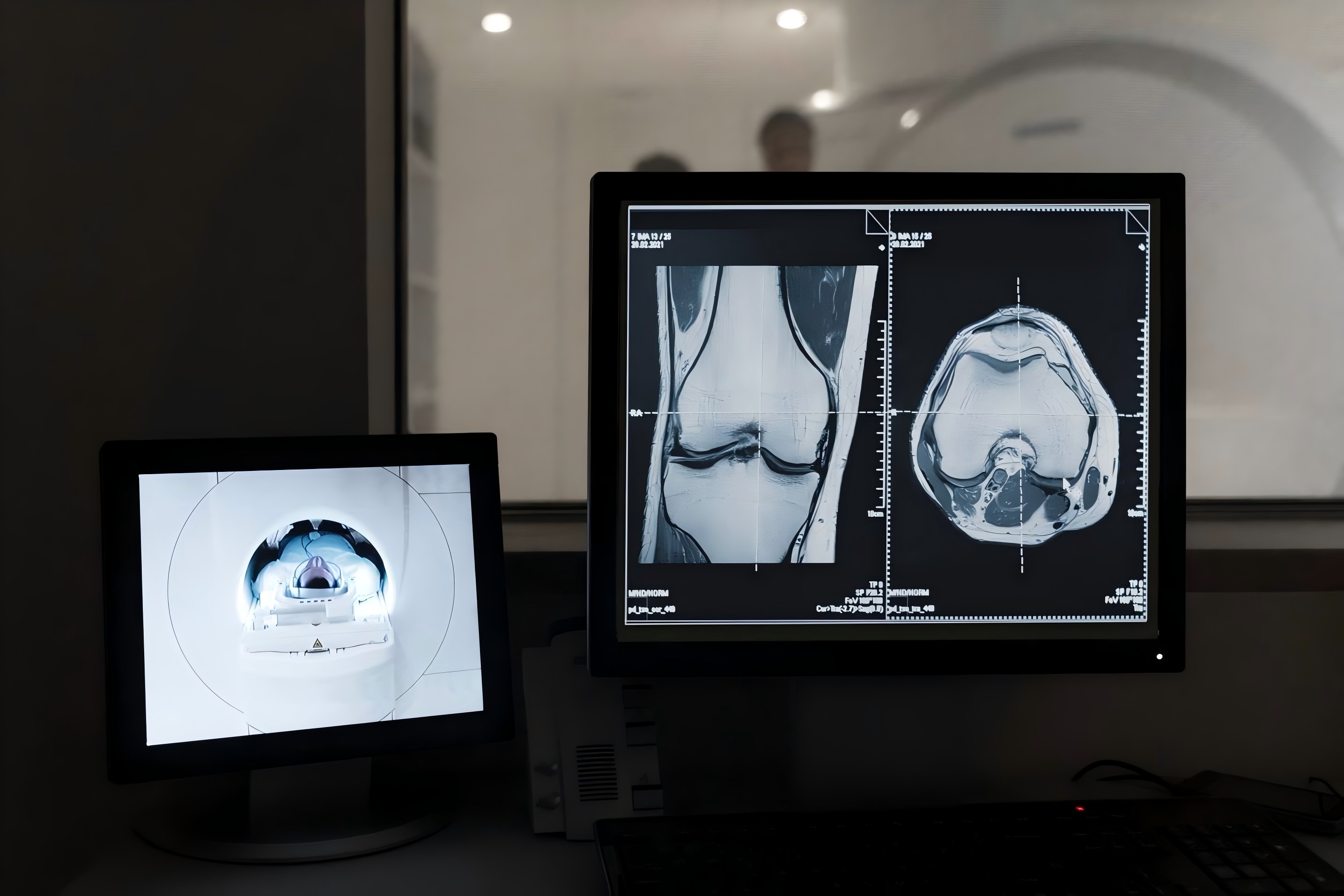
Bone metastases represent a critical diagnostic challenge in oncology, often signaling advanced disease and influencing treatment strategy, prognosis, and quality of life. Traditional methods of detection via computed tomography (CT) can be time-intensive and subject to inter-reader variability, particularly in cases involving small or atypically located lesions. In recent years, artificial intelligence (AI) has emerged as a powerful tool to enhance the sensitivity, efficiency, and reproducibility of radiologic assessments. Among these innovations, a clinically applicable AI system designed to detect and diagnose bone metastases (BMs) using CT scans is now gaining traction for its real-world potential.
Such systems are developed through rigorous model training pipelines based on large-scale, annotated CT datasets derived from retrospective cohorts or multicenter collaborations. These datasets include volumetric CT scans from confirmed cases of bone metastases, with lesion annotations verified through biopsy, pathology reports, or longitudinal follow-up imaging to ensure gold-standard diagnostic accuracy. The AI architecture typically leverages cutting-edge deep learning models—such as three-dimensional convolutional neural networks (3D CNNs) and transformer-based frameworks like Swin Transformers—which can process complex spatial data and recognize patterns across multiple skeletal regions, including the spine, pelvis, and ribs. Importantly, these models are designed to reflect the multifocal nature of skeletal metastases, a key challenge in clinical detection.
To enhance clinical interpretability, visualization tools such as Grad-CAM heatmaps are incorporated, allowing clinicians to identify the regions of the CT scan most influential in the model’s decision-making. This transparency improves confidence and supports integration into diagnostic workflows. Validation studies have demonstrated strong performance, with lesion-wise sensitivities approaching 89% and classification accuracies exceeding 90% across diverse test sets. The combination of high accuracy, scalability, and interpretability positions these AI tools as promising adjuncts in the diagnostic arsenal for bone metastases.1,2,3,4
Model Development: From Data to Diagnosis
The training of a clinically deployable AI system for bone metastasis detection begins with curating comprehensive, well-labeled CT datasets. These are typically collected from multiple institutions and encompass a variety of scanner types and clinical settings to ensure heterogeneity and generalizability. Each CT scan includes precise lesion annotations, verified against histopathology or serial imaging follow-up, covering a spectrum of metastatic types (osteolytic, osteoblastic, mixed) and anatomical locations.
Advanced deep learning architectures form the backbone of the detection model. Three-dimensional CNNs provide a natural fit for volumetric CT data, enabling the system to learn spatial features that extend beyond what 2D slices can capture. Transformer-based models, particularly Swin Transformers, offer additional advantages by incorporating long-range contextual relationships and attention mechanisms, further refining lesion detection and classification.
Beyond detection, these systems aim for clinical realism by supporting multi-site lesion identification across the entire skeleton. Given the diffuse nature of metastatic spread, comprehensive whole-body screening is essential. To support explainability—a key requirement for clinical acceptance—visual interpretability tools such as Grad-CAM and saliency maps are integrated into the system, highlighting scan regions that contribute most strongly to predictions. This visual feedback aids radiologists in verifying AI-generated results and facilitates more informed diagnostic decisions.
Performance metrics from recent studies validate the robustness of these approaches. AI systems trained using this methodology routinely achieve lesion-wise sensitivity near 89%, with classification accuracies above 90% on internal and external test sets. These results not only demonstrate the diagnostic power of AI but also reflect its potential to augment radiologists’ performance, particularly in detecting subtle or small-volume metastatic lesions.5,6,7
Clinical Validation and Real-World Application
One of the most thoroughly validated AI tools in this domain is the Bone Lesion Detection System (BLDS), tested in a multicenter cohort comprising 2,518 patients across five hospitals. The system achieved a lesion-wise sensitivity of 89.1%, patient-wise sensitivity of 90.2%, and a negative predictive value of 98.2% during validation involving over 54,000 patients. These results reflect high diagnostic precision, with only 1.40 false positives per case—an important metric for minimizing unnecessary follow-up and overdiagnosis.
In a randomized crossover study, BLDS improved radiologists’ lesion detection sensitivity by 22.2% and reduced average reading time by 26.4%. It also demonstrated superiority over junior radiologists and matched the performance of senior trainees, suggesting a valuable role in training and quality assurance. Importantly, the system is capable of differentiating between various bone lesion types—including osteoblastic, osteolytic, mixed, and benign lesions—without requiring pathological confirmation, thus streamlining the diagnostic process.
BLDS has been prospectively implemented across emergency, outpatient, and inpatient workflows, using CT scanners from 13 different manufacturers. This level of deployment underscores the system’s adaptability to real-world conditions and diverse imaging equipment. Unlike many experimental models that remain confined to research settings, this AI tool has been fully integrated into Picture Archiving and Communication Systems (PACS), functioning as a seamless part of routine radiologic workflows.
Systematic reviews continue to highlight the scarcity of AI models for bone metastasis detection that are both clinically validated and prospectively deployed. The BLDS represents a rare exception—bridging the gap between experimental proof-of-concept and clinical-grade utility. Its high accuracy, robust real-world validation, and ease of integration signal a paradigm shift in how AI can assist oncologic imaging, particularly in the context of complex, system-wide metastasis detection.8,9
Reference:
1. Zhang Y, Li J, Yang Q, Yin S, Hou J, Cao X, Ma S, Wang B, Luo M, Zhou F, Xu J, Wang S, Wu Y, Zhang J, Luo X, Yang Z, Ma W, Lin D, Zhan Y, Zhou XS, Yu X, Shen D, Zhang R, Xie C. A clinically applicable AI system for detection and diagnosis of bone metastases using CT scans. Nat Commun. 2025 May 13;16(1):4444. doi: 10.1038/s41467-025-59433-7. PMID: 40360470; PMCID: PMC12075757.
2. Belue MJ, Harmon SA, Yang D, An JY, Gaur S, Law YM, Turkbey E, Xu Z, Tetreault J, Lay NS, Yilmaz EC, Phelps TE, Simon B, Lindenberg L, Mena E, Pinto PA, Bagci U, Wood BJ, Citrin DE, Dahut WL, Madan RA, Gulley JL, Xu D, Choyke PL, Turkbey B. Deep Learning-Based Detection and Classification of Bone Lesions on Staging Computed Tomography in Prostate Cancer: A Development Study. Acad Radiol. 2024 Jun;31(6):2424-2433. doi: 10.1016/j.acra.2024.01.009. Epub 2024 Jan 22. PMID: 38262813; PMCID: PMC11214604.
3. Li W, Zou X, Zhang J, Hu M, Chen G, Su S. Predicting lung cancer bone metastasis using CT and pathological imaging with a Swin Transformer model. J Bone Oncol. 2025 Apr 17;52:100681. doi: 10.1016/j.jbo.2025.100681. PMID: 40342492; PMCID: PMC12059344.
4. van der Linden LR, Vavliakis I, de Groot TM, Jutte PC, Doornberg JN, Lozano-Calderon SA, Groot OQ. Artificial Intelligence in bone Metastases: A systematic review in guideline adherence of 92 studies. J Bone Oncol. 2025 Apr 24;52:100682. doi: 10.1016/j.jbo.2025.100682. PMID: 40337637; PMCID: PMC12056386.
5. Belue MJ, Harmon SA, Yang D, An JY, Gaur S, Law YM, Turkbey E, Xu Z, Tetreault J, Lay NS, Yilmaz EC, Phelps TE, Simon B, Lindenberg L, Mena E, Pinto PA, Bagci U, Wood BJ, Citrin DE, Dahut WL, Madan RA, Gulley JL, Xu D, Choyke PL, Turkbey B. Deep Learning-Based Detection and Classification of Bone Lesions on Staging Computed Tomography in Prostate Cancer: A Development Study. Acad Radiol. 2024 Jun;31(6):2424-2433. doi: 10.1016/j.acra.2024.01.009. Epub 2024 Jan 22. PMID: 38262813; PMCID: PMC11214604.
6. Papalia, G.F.; Brigato, P.; Sisca, L.; Maltese, G.; Faiella, E.; Santucci, D.; Pantano, F.; Vincenzi, B.; Tonini, G.; Papalia, R.; et al. Artificial Intelligence in Detection, Management, and Prognosis of Bone Metastasis: A Systematic Review. Cancers 2024, 16, 2700. https://doi.org/10.3390/cancers16152700
7. Tao H, Hui X, Zhang Z, Zhu R, Wang P, Zhou S, Yang K. Accuracy of artificial intelligence in detecting tumor bone metastases: a systematic review and meta-analysis. BMC Cancer. 2025 Feb 18;25(1):286. doi: 10.1186/s12885-025-13631-0. PMID: 39966724; PMCID: PMC11837447.
8. Zhang S, Zhu Z, Yu Z, Sun H, Sun Y,Huang H, Xu L, Wan J. Effectiveness of AI for Enhancing Computed Tomography Image Quality and Radiation Protection in Radiology: Systematic Review and Meta-Analysis. J Med Internet Res 2025;27:e66622
9. Lin, Q., He, Y., Guo, S.: AI-powered automated analysis of bone scans: A survey. IET Image Process. 19, e13311 (2025). https://doi.org/10.1049/ipr2.13311






Post comments